What are chlorosilanes?
Methyl-substituted chlorosilanes are prepared by the reaction between silicon powder and methyl chloride, and are the basic building blocks for a vast array of silicone substances.
Chlorosilanes are chemical compounds prepared by reacting silicon powder and methyl chloride (CH3CI) gas. The methyl chlorosilanes produced (trimethyl, dimethyl and methyl chlorosilane) are the building blocks for various silicone substances.
From silicon to chlorosilanes:
Production of silicones, firstly requires the synthesis of their precursors: chlorosilanes. These are prepared by reacting silicon powder (extracted from natural silica by means of an electrochemical process) and CH3CI or methyl chloride gas (formed in the reaction between HCI and CH3OH or methanol).
This reaction is called direct synthesis, and it is performed in large-scale reactors and produces a crude mixture of several liquid chlorosilanes which must then be separated by distillation.
The chlorosilanes produced are the building blocks with which the various silicone substances are then produced: the central silicon atom is bonded to various numbers of chlorine atoms, each of which provide a potential reactive site for the hydrolysis reaction that enables the length and branching of the polymer molecular framework to be controlled.
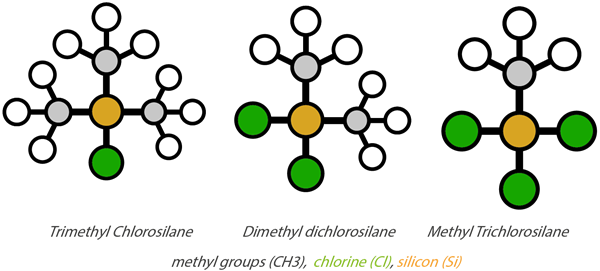
The three types of methyl chlorosilanes obtained during direct synthesis which are then used to produce silicones are: trimethyl chlorosilane, dimethyl dichlorosilane and methyl trichlorosilane, as shown in the diagram below.
From chlorosilanes to basic silicone products:
Chlorosilanes undergo hydrolysis and polycondensation to give the basic silicone products: oils, gums, and resins. Oils and gums are straight-chain compounds that are either reactive or non-reactive. When the compounds are fluid in consistency they are considered as oils, whereas when their viscosity increases to the point of solidifying, they are considered to be gums.
Oils and gums can also be subject to further physical and chemical processing at a later stage. Resins have a branched structure that forms molecular lattices. These basic silicone products have a molecular framework based around the four different groups that result from the original chlorosilanes.
Hydrolysis of chlorosilanes to form the siloxane backbone
Hydrolysis produces short-chain polymers which must then be lengthened to give the required molecular weight. Pure dimethyl dichlorosilanes are hydrolyzed to obtain oils. Resins are obtained by hydrolysis of a mixture of dimethyl dichlorosilanes and mathyl trichlorosilanes, to which solvents are added to avoid gelling.
Lengthening of polymer chains by polycondensation
Polycondensation of prepolymers is performed using catalysts. The addition of chain terminators enables the polymer chain ends to be de-activated i.e. to give non-reactive oils. To prepare certain specialty silicone products with special properties, it is sometimes necessary to modify the CH3 (non-reactive) groups that are initially bonded to the silicon atoms. Therefore, these groups are partially replaced by other non-reactive groups (-C6H5, etc.) or reactive groups (-H, CH=CH2, etc.) in certain cases, prior to or following hydrolysis.
Contact us
Take your business to the next level by partnering with a world-leading material manufacturer.